Research
Contents
- Importance of plant reproduction studies |![]() read
read
- Importance of meiosis studies in plants |![]() read
read
- An outline of Nonomura lab researches |![]() read
read
- Germline initiation/development and fate determination as meiotic cells |![]() read
read
- An Argonaute protein essential for meiosis progression |![]() read
read
- An RRM protein regulating proper timing of mitosis-to-meiosis transition in rice |![]() read
read
- Genes regulating differentiation/development of reproductive tissues in rice |![]() read
read
- Molecular mechanisms of SAM development focusing on KNOX transcription factor
|![]() read
read
Importance of plant reproduction studies
Seeds and fruits are just the "fruits" of plant reproduction. That is, to elucidate the regulatory mechanisms of plant reproduction is an important theme not only for basic researches, but also for applied studies in efficient food production and breeding methods.
Most organisms transfer genetic information precisely via reproductive processes, and furthermore generate genetic diversity by meiotic recombination. To simultaneously achieve these contrary purposes, genome stability and genetic diversity, reproductive processes are regulated by complicated and highly organized mechanisms.
Reproduction in angiosperms starts from transition of shoot apical meristem to inflorescent, which is followed by development of inflorescent and floral organs, differentiation of germline cells, meiosis, developemnt of gametes, and fertilization. To date, the processes from meristem transition to flower development, and from gamete development to fertilization, have been well studied by many plant researchers.
Importance of meiosis studies in plants
Meiosis is a pivotal event to generate and transfer genetic diversity to the next generation via meiotic recombination. In addition, to achieve accurate meiotic recombination, each chromosome recognize and associate with a homologous counterpart during meiosis I, called "homologous pairing”.
Germline development and meiosis have not been studied well in plants, compared to animal studies. It may be since sampling of reproductive organs is relatively difficult, and also since genetic analyses are troublesome because of relating mutations often resulting in infertility. Furthermore, different from development of reproductive organs and gametephytes, rich in plant-specific events, everyone believes that plant meiosis studies cannot give any knowledge beyond that in advanced yeast study. It is partially true, but on the other hand, we and other groups have unveiled that there are many plant-specific mechanisms in plant meiosis and reproductive organ development.
In addition, it is reported one after the other that genome-wide structural alteration/modification of chromatins takes place during mitosis-to-meiosis transition in many organisms. Drastic alteration of meiotic chromosome localization in meiotic nuclei is as well. These suggest that in addition to functions of structural and regulatory genes, epigenetic gene functions in chromatin modification and chromosome kinetics are essential in meiotic processes. Nonomura lab aims to contribute to revealing biological meanings of epigenetic regulation trough plant meiosis study.
Meiotic events also relate closely to establishment of biological species. If gene alignment and composition are same, even though chromosome structure and numbers are different, between parents, their child can live his/her natural life span. However, differences in chromosome number and structure cause in aberrant meiotic recombination and pairing, often resulting in infertility. This kind of infertility can be attributable to "reproductive isolation", and parental individuals are defined as different species with each other. We can find the "reproductive isolation"-dependent infertility in hybrids between cultivars and related wild species. There are many reports showing that one of the reasons for such infertilities is found in meiotic defects.
If regulatory mechanisms driving meiosis were elucidated, we think that it will be able to freely control efficiency and chromosomal positions of meiotic recombination, and to efficiently introduce useful genes from wild species to cultivars.
An outline of Nonomura lab researches
We are mainly studying in genetics of early germline development, meiosis, and developmental processes regarding reproductive events, using rice as a model cereal in monocotyledonous plants. Of rice genes identified through mutant analyses so far, we focus on the genes which are expected to bring new findings in genetic and epigenetic phenomena specific to plants, and aim to original researches in plant reproductive development and meiosis, clearly different from studies in yeast and animals.
(1) Molecular cytogenetics on plant reproductive processes in early germline development and meiosis.
(2) Developmental genetics regarding plant reproduction.
(3) Improvement of plant breeding efficiency by manipulating meiotic genes.
Germline initiation/development and fate determination as meiotic cells
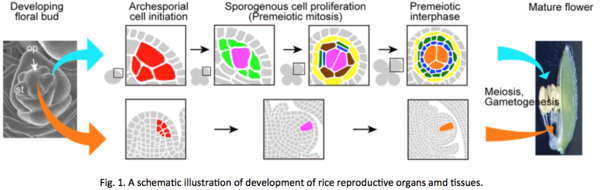
A plant cell in which the cell fate is firstly determined as the germline is called "archesporial cell" in plants (Fig. 1). The mechanisms determining the cell fate of archesporial cells have been hardly identified.
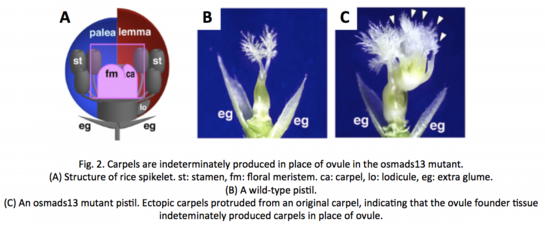
Rice gene OsMADS13 is homologous to Arabidopsis AGAMOUS (AG), that functions in anther and pistil development, and plays essential roles in floral meristem determination and ovule development. In osmads13 mutants, though the carpel that forms the outward appearance of pistil is developed normally, ectopic pistils are indeterminately formed in place of the ovule, developed inside the pistil (Fig. 2). Our results suggest that floral meristem determination is required for development of ovules and female primordial germ cells, and also that OsMADS13 functions in such processes.
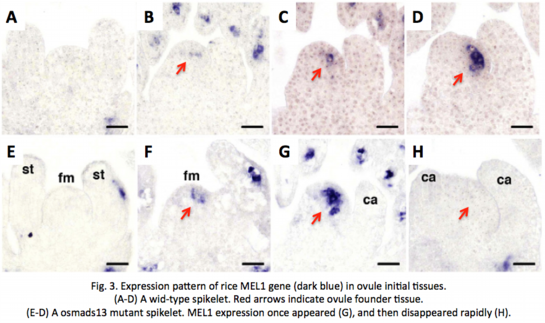
As mentioned above, the ovule is replaced by ectopic carpels in osmads13 mutants. Thus, to prove that no female germline is differentiated in the mutant, we investigated the germline-specific expression of MEL1 gene (see below) in spikelets. Surprisingly, however, MEL1 expression appeared once for a short time in the archesporial cell-initiation stage, and disappeared rapidly in subsequent stages (Fig. 3, ![]() Yamaki et al. 2011).
Yamaki et al. 2011).
MEL1 is known as a germline-specific gene/protein responsible for normal meiosis progression (see below, ![]() Nonomura et al. 2007). So, the result strongly suggest the following possibilities; (1) cell-fate determination for meiosis begins at the archesporial cell-initiation stage, (2) The regulatory mechanism to initiate MEL1 gene expression functions independently of the OsMADS13-dependent mechanism determining the ovule fate, and (3) normal development of the ovule is required for maintenance of MEL1 gene expression.
Nonomura et al. 2007). So, the result strongly suggest the following possibilities; (1) cell-fate determination for meiosis begins at the archesporial cell-initiation stage, (2) The regulatory mechanism to initiate MEL1 gene expression functions independently of the OsMADS13-dependent mechanism determining the ovule fate, and (3) normal development of the ovule is required for maintenance of MEL1 gene expression.
We'd like to unveil a part of molecular mechanisms regulating differentiation of plant archespores via MEL1-promoter analysis, and so on.
Analysis of a rice Argonaute protein essential for meiosis progression
We analyzed one of rice mutants exhibiting a seed sterile phenotype, and named the causal gene as the MEIOSIS ARRESTED AT LEPTOTENE1 (MEL1) gene (![]() Nonomura et al. 2007). MEL1 encodes an Argonaute protein (AGO) that animals and plants commonly conserve. AGO makes a complex with small RNA, and the complex regulates translation and transcription in a small RNA-dependent manner. The loss-of-function mutation of rice MEL1 gene causes arrested meiosis at leptotene stage, resulting in complete seed sterility.
Nonomura et al. 2007). MEL1 encodes an Argonaute protein (AGO) that animals and plants commonly conserve. AGO makes a complex with small RNA, and the complex regulates translation and transcription in a small RNA-dependent manner. The loss-of-function mutation of rice MEL1 gene causes arrested meiosis at leptotene stage, resulting in complete seed sterility.
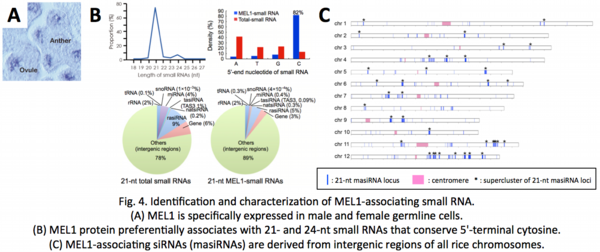
To reveal the MEL1 molecular function, we immunoprecipitated and deep-sequenced MEL1-associating small RNAs (masiRNAs) (![]() Komiya et al. 2014;
Komiya et al. 2014; ![]() Komiya and Nonomura 2014) . As a result, 75% of masiRNAs were in 21-nucleotides (nt) long, 80% of which conserved the cytosine at the 5'-terminus. Surprisingly, masiRNAs were derived from 1,000 or more loci on intergenic, protein noncoding regions of rice chromosomes, and 700 of more species of long noncoding RNAs (lncRNAs) were transcribed from those loci specifically in reproductive organs. This suggests that the lncRNAs are the precursors of masiRNAs. However, it has been difficult to narrow down the candidates targeted by the MEL1/masiRNA complexes, due to huge numbers of masiRNA species.
Komiya and Nonomura 2014) . As a result, 75% of masiRNAs were in 21-nucleotides (nt) long, 80% of which conserved the cytosine at the 5'-terminus. Surprisingly, masiRNAs were derived from 1,000 or more loci on intergenic, protein noncoding regions of rice chromosomes, and 700 of more species of long noncoding RNAs (lncRNAs) were transcribed from those loci specifically in reproductive organs. This suggests that the lncRNAs are the precursors of masiRNAs. However, it has been difficult to narrow down the candidates targeted by the MEL1/masiRNA complexes, due to huge numbers of masiRNA species.
Recently, we revealed by immunofluorescent staining that when wild-type rice germ cells transit from mitosis to meiosis, the histone H3s of meiotic chromosomes are dimethylated at 9th lysine (H3K9) genome-widely, and that demethylated at the end of meiosis I (![]() Liu and Nonomura 2016). This “Large-scale Meiotic Reprogramming (LMR)", named by our group, takes place dependent on MEL1 function, according to the results of mel1 mutant analyses.
Liu and Nonomura 2016). This “Large-scale Meiotic Reprogramming (LMR)", named by our group, takes place dependent on MEL1 function, according to the results of mel1 mutant analyses.
Furthermore, a possibility that a subset of masiRNAs are produced in anther tapetum, somatic cell layers surrounding male germ cells, also emerges from our preliminary results. A transcription factor that plays an essential role in tapetum development also activates transcription of multiple genes required for masiRNA precursor transcription (Ono et al. in preparation). This result implies that reproductive small RNA acts as an intercellularlly mobile signal from soma to germline cells.
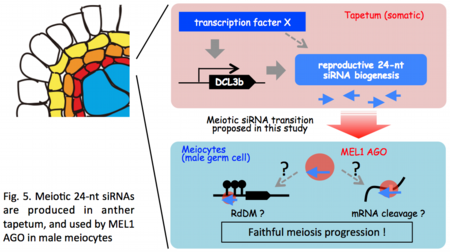
MEL1 protein is suggested to move from the cytoplasm to the nucleus in germ cells during mitosis-to-meiosis transition (![]() Komiya et al. 2014). Thus, we'd like to unveil the MEL1 molecular function(s) via identification of MEL1-interacting small RNAs/proteins and MEL1 subcellular localization separably in different premeiotic or meiotic cell stages.
Komiya et al. 2014). Thus, we'd like to unveil the MEL1 molecular function(s) via identification of MEL1-interacting small RNAs/proteins and MEL1 subcellular localization separably in different premeiotic or meiotic cell stages.
Analyses of an RRM protein regulating proper timing of mitosis-to-meiosis transition in rice
Developmental processes of plant germline are strictly regulated in angiosperms, to complete seed production during a short limited season. Synchronous male meiosis within a same anther, resulting in simultaneous production of pollen grains, is an example. Our group previously identified a rice protein, MEL2, regulating proper timing of meiosis transition (![]() Nonomura et al. 2011). However, the mechanisms are largely ambiguous yet.
Nonomura et al. 2011). However, the mechanisms are largely ambiguous yet.
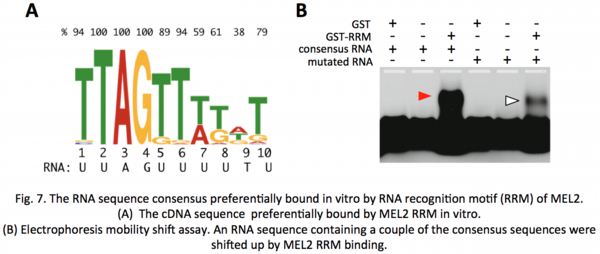
Recently, we identified RNA consensus sequences that MEL2 preferentially binds in vitro (![]() Miyazaki et al. 2015). Searched for the rice genome, the consensus-like sequences were frequently found in 3'-untranslated regions (3'-UTR) of 249 genes. Proteomic analyses revealed that several proteins that altered their amounts in mel2 mutant anthers were supposed to function in meiosis, and that the 3'-UTRs of their corresponding mRNAs contained MEL2-binding consensus-like sequences. These results suggest that MEL2 regulates meiosis transition via translational control of meiotic target mRNAs.
Miyazaki et al. 2015). Searched for the rice genome, the consensus-like sequences were frequently found in 3'-untranslated regions (3'-UTR) of 249 genes. Proteomic analyses revealed that several proteins that altered their amounts in mel2 mutant anthers were supposed to function in meiosis, and that the 3'-UTRs of their corresponding mRNAs contained MEL2-binding consensus-like sequences. These results suggest that MEL2 regulates meiosis transition via translational control of meiotic target mRNAs.
Now we are aiming to capture and sequence RNAs in vivo targeted by MEL2, and to reveal a part of the mechanisms regulating proper timing of mitosis-to-meiosis transition in rice.
Analyses of genes regulating differentiation and development of reproductive organs/tissues in rice
The anther is a sporophytic organ containing diploid germ cells that undergo meiosis and produce haploid microspores. It is of four lobes, and in each lobe, sporophytic male germ cells are aligned at a central axis, and surrounded by somatic cell layers (Fig. 8). Tapetum is the innermost somatic-cell layer adhering to central meiocytes, and its role in post-meiotic pollen development has been well documented. Despite of its roles also supposed in early meiosis, the molecular entity supporting meiotic interaction between soma and germ cells is largely ambiguous.
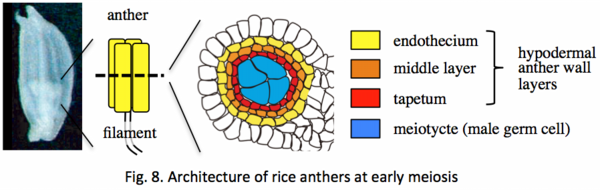
I identified the rice reproductive gene, MSP1, encoding a receptor-like protein kinase (![]() Nonomura et al. 2003). MSP1 determined the fate of hypodermal anther wall layers, whose ability to be germ cells was suppressed by MSP1 function. MSP1 protein is an LRR receptor-like serine/threonine protein kinase having a membrane-transfer domain at the middle. The results also suggested that normal anther-wall development is required for proper meiosis progression. The results from another group identified a short-peptide ligand OsTDL1a bound by the MSP1 receptor (
Nonomura et al. 2003). MSP1 determined the fate of hypodermal anther wall layers, whose ability to be germ cells was suppressed by MSP1 function. MSP1 protein is an LRR receptor-like serine/threonine protein kinase having a membrane-transfer domain at the middle. The results also suggested that normal anther-wall development is required for proper meiosis progression. The results from another group identified a short-peptide ligand OsTDL1a bound by the MSP1 receptor (![]() Zhao et al. 2008). This work is pioneer in anther developmental research in grass species.
Zhao et al. 2008). This work is pioneer in anther developmental research in grass species.
Recently, we found that a transcription factor that plays an essential role in tapetum development also activates transcription of multiple genes required for masiRNA precursor transcription (Ono et al. in preparation). This result implies that reproductive small RNA acts as an intercellularlly mobile signal from soma to germline cells, and also supports the results in a previous MSP1 study (![]() Nonomura et al. 2003). Cell-to-cell signaling networks in reproductive organs is one of our important research issunes.
Nonomura et al. 2003). Cell-to-cell signaling networks in reproductive organs is one of our important research issunes.
Molecular mechanisms of SAM development focusing on KNOX transcription factor
In contrast to animals that usually complete organogenesis early in development, plants continue to produce organs throughout the life cycle. The shoot apical meristem (SAM) is responsible for the continuous production of all aboveground organs in flowering plants. Since plants are sessile organisms and cannot move away from danger, they must adapt to their environment. The SAM is central to the decision-making processes concerning identity, timing and number of organs based on environmental conditions. This aspect is agronomically important as well. Our goal is to understand the behavior of the SAM at the molecular levels under various developmental and environmental contexts.
The SAM is an indeterminate structure composed of self-renewing stem cells in its center and their daughter cells at the periphery. The indeterminacy of the SAM is maintained by the homeobox gene knotted1 (kn1) in maize. kn1 is expressed throughout the SAM, but is down-regulated at the flank of the SAM where lateral organs such as leaves and flowers (Fig. 9). Ectopic expression of kn1 in leaves disturbs leaf differentiation, whereas its loss-of-function impairs SAM maintenance.
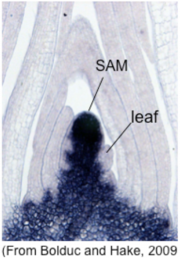
Fig. 9. in situ localization of KNOTTED1 transcript
Positive autoregulation of a rice KNOX gene OSH1
SAM-specific expression of KNOX genes is crucial for plant development; however, the molecular mechanism that maintains their expression was largely unknown. Previously, we isolated a loss-of-function mutant of Oryza sativa homeobox1 (OSH1), a rice ortholog of maize kn1. Among five KNOX genes in rice, only loss-of-function mutants of OSH15 had been reported. Genetic analysis revealed that OSH1 is predominantly required for SAM maintenance (Figure 10A and B) and that OSH1 and OSH15 have redundant functions in SAM establishment in embryos. Expression analysis in these mutants showed that KNOX gene expression is positively regulated by the phytohormone cytokinin (CK) and by their own function, indicating the presence of positive autoregulatory loops. Chromatin immunoprecipitation (ChIP) and gel-shift assay revealed that OSH1 directly activates all five KNOX genes through evolutionarily conserved cis-elements. Mutations in these cis-elements in the OSH1 locus abolished GFP-OSH1 expression (Figure 10C and D). These results showed that the positive autoregulation of OSH1 is an indispensable mechanism for self-maintenance of the SAM (Figure 10E) (![]() Tsuda et al. 2011).
Tsuda et al. 2011).
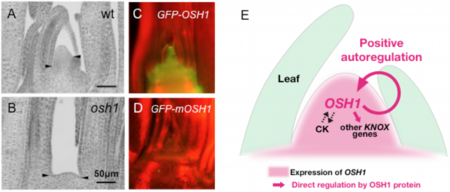
Fig. 10. Positive autoregulation of OSH1 is important for its expression
(A, B) osh1 mutants can not maintain the SAM.
(C, D) Mutations in OSH1 binding sites abolishes GFP-OSH1 expression.
(E) OSH1 autoregulatory loops for SAM maintenance.
OSH1 represses BR pathway by activating BR catabolism genes
Next, we sought important downstream pathways of OSH1. Analysis of OSH1 inducible overexpression (35S:OSH1-GR) phenotypes revealed that the induction caused insensitivity to brassinosteroid (BR), a phytohormone that promotes differentiation by activating genes related to cell elongation and cell wall modification (Figure 11A).
ChIP-seq using anti-OSH1 antibodies coupled with RNA-seq analyses of 35S:OSH1-GR revealed that 391 direct targets were up/down-regulated after OSH1 induction (Figure 11B). As is the case of maize KN1 targets, OSH1 targets are enriched with transcription factors and hormone pathway genes. Among them, three genes encoding BR catabolism enzymes were rapidly activated after OSH1 induction (Figure 11C). RNAi knockdown of these genes mimicked some of osh1 phenotypes (Figure 11D). These observations highlighted BR inactivation as an important function of OSH1 to maintain SAM activity (Figure 11E) (![]() Tsuda et al. 2014).
Tsuda et al. 2014).
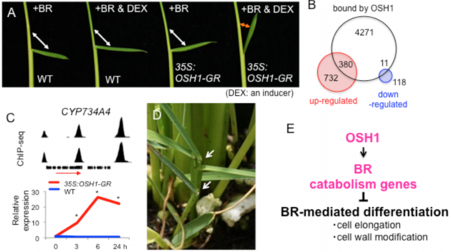
Fig. 11. OSH1 directly activate BR catabolism genes
(A) Induced overexpression of OSH1 caused BR insensitivity in leaf angle test.
(B) Number of OSH1-bound and -modulated genes.
(C) An example of BR catabolism gene, CYP734A4, directly activated by OSH1.
(D) CYP734S RNAi lines showed the arrested shoot growth, similar to oohs mutants.
(E) A model of the repression of BR-mediated cell differentiation by OSH1.
Ongoing Projects
Our ChIPseq study revealed that OSH1 binds more than 4000 loci in the rice genome. Putative targets include various hormone pathways such as auxin, BR, gibberellin, cytokinin and ethylene, as well as many transcription factors involved in plant development. To understand how OSH1 regulates them, our current projects focus on the identification and characterization of KNOX cofactors such as BLH family homeodomain proteins.
Monocot stems have an unique anatomy (such as scattered veins and the lack of vascular cambia) and a great agronomical importance for plant height regulaiton. We recently found that KNOX and BLH genes play crucial roles the development of inner stem structures, such as the intercalary meristems and vascular bundles (Tsuda et al. unpublished). By studying developmental function of KNOX genes combined with their downstream pathways, we aim to tweek plant architectures for the improved crop production.